The trypanosomiases are a disease complex that affects man and his livestock in Africa, Asia and South America. ILRAD is concerned with those trypanosomiases transmitted by tsetse flies, which threaten the lives of some 30% of an estimated population of 150 million cattle in 37 countries of Africa, as well as comparable numbers of small ruminants. Annual losses in meat production alone are estimated at US$5 billion. This economic deprivation is exacerbated by losses in milk production, tractive power, waste products that provide natural fuel and fertilizer and secondary products such as clothing and hides. In addition, 50 million people are currently exposed to the risk of contracting human trypanosomiasis, known as sleeping sickness.
Trypanosomiasis is caused by blood-dwelling protozoan parasites called trypanosomes, which infect man, cattle, sheep, goats, pigs, horses and camels. Wild animals can also be infected and serve as reservoirs of infection. Trypanosomes are transmitted by tsetse flies (Glossinidae) and other biting insects, which ingest the parasites in blood meals taken from infected animal hosts (Figure 12). In the tsetse fly the parasites multiply in the midgut or in the proboscis and undergo a developmental cycle culminating in the generation of metacyclic trypanosomes, which are infective to the mammalian host. Metacyclic forms are introduced into a new host through the saliva of the tsetse fly during feeding. In the mammalian host the parasites develop in the tissues and blood, causing anaemia, reduced productivity, reproductive disorders and death.
Figure 12. Schematic diagram showing the life cycles of the three major tsetse-transmitted trypanosome species in Africa.
Three major species of trypanosomes of veterinary importance are studied at ILRAD. These are Trypanosoma congolense, Trypanosoma vivax and Trypanosoma brucei brucei. Biological aspects of Trypanosoma evansi, which belongs to the same Trypanozoon group as T. brucei but which is transmitted by biting flies other than tsetse, are also studied at ILRAD. Trypanosoma simiae, which is closely related to T congolense, is primarily a parasite of pigs but is frequently found in trypanosome isolates taken from ruminants and tsetse flies in the field. Two further subspecies of T. brucei, T. brucei rhodesiense and T. brucei gambiense infect man and are reservoirs of infection in various types of wild animals.
In addition to there being many species of trypanosomes, a phenomenon called antigenic variation exhibited by each species helps to induce chronic infections in the hosts the parasites infect. Trypanosomes are covered by a dense coat made up of glycoprotein molecules, called variable surface glycoproteins (VSGs) (Figure 13). A trypanosome population causing infection in a single susceptible host animal can vary these surface antigens in a matter of a few days. Although the host usually generates a good antibody response to trypanosomes expressing a particular variable antigenic type (VAT), the large number of VATs that can be expressed within a single infection, estimated to be 300-1000 for T. brucei, causes chronic infections. Long-term infections in turn help ensure the transmission of the parasites to other animals and increase the severity of the pathogenesis, particularly anaemia, caused by trypanosome infection.
Figure 13. Schematic diagram of a trypanosome of the Trypanosoma brucei group in its intermediate bloodstream form, illustrating the major organelles.
It was initially believed that study of the antigenic variation displayed by trypanosomes would best elucidate how trypanosomes maintain themselves in the host animal. This information could then be used to develop vaccines to control or prevent the infection. But detailed studies of antigenic variation carried out at ILRAD and elsewhere over the last several years suggest that the number of VSGs expressed by trypanosomes is too great to make a vaccine based on the administration of a single, or even a few, VATs broadly effective. The total number of antigens expressed by any stock of trypanosomes, called a repertoire, is very large. These antigenic repertoires vary not only among but also within species: isolates in one area of Africa often differ in their antigenic repertoire from isolates in other areas. These distinct antigenic repertoires of a single species are known as serodemes. The existence of serodemes considerably enlarges the number of VATs encountered in the field.
Traditional management of trypanosomiasis has relied on chemotherapeutic drugs to treat infections in livestock and on insecticides to control the tsetse vector. Because the widespread use of insecticides is environmentally damaging, attempts have recently been made to control the disease by combining the strategic use of insecticide-impregnated traps and screens, to reduce the tsetse populations, with chemotherapeutic treatment for the animals that succumb to the lowered challenge. This approach, when followed by close monitoring of the tsetse population and the disease status of livestock, has successfully controlled trypanosomiasis in a few trial areas, but these methods are too difficult to apply in most parts of Africa. Furthermore, relying on one or two drugs to treat infected livestock increases the possibility that the parasites will develop resistance to the drugs.
ILRAD is therefore taking an alternative approach and is seeking primarily immunological solutions to the problems posed by trypanosomiasis. The complexity of the antigenic variation exhibited by the parasite has necessitated that the research goals of the trypanosomiasis program be divided into short and long term. In the short term ILRAD is studying ways to develop more accurate tests to diagnose trypanosome infection in livestock, new ways with which to use the currently available trypanocides that will reduce the possibility of inducing parasite resistance to the drugs, and the reasons why some breeds of cattle are able to withstand trypanosome infection better than others. In the long term ILRAD is conducting research in two main areas. It is studying the responses ruminant hosts make to trypanosome infection, with the aim of enhancing normal mechanisms of resistance in livestock, and it is scrutinizing molecules and processes of the parasite in a search for key elements or activities that can be attacked with drugs without adversely affecting the host.
Short-term strategies for trypanosomiasis control
Tests to diagnose infections
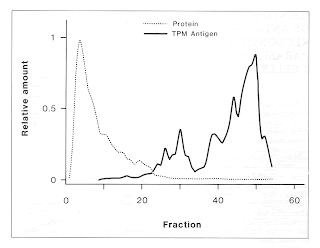
To detect and identify trypanosome infections in tsetse flies, domestic livestock and man, ILRAD scientists for several years have been developing diagnostic tests that use monoclonal antibodies in enzyme-linked immunosorbent assays (ELISAs). Reagents are also available to identify trypanosomes of the subgenera Trypanozoon, Nannomonas and Duttonella. In 1988 new monoclonal antibodies were developed to identify protein rather than carbohydrate epitopes of T. vivax and T. congolense membrane proteins. These antibodies will reduce the likelihood of the ELISA giving false positive results. Furthermore, by detecting target proteins, the antibodies will enable scientists to identify fragments of DNA encoding the trypanosomal antigens in libraries of complementary DNA-DNA copied from messenger RNA of the various trypanosome species. Recombinant antigens could then be synthesized in great quantity for use in inhibition ELISAs, which may be more sensitive in detecting infections than the technique presently used. In 1988 the trypanosomiasis program began to investigate the synthetic production of the diagnostic antigen for T. brucei.
The assays in their present form are now being tested for accuracy and sensitivity in national laboratories in ten African countries in collaboration with the World Health Organization and a joint division of the Food and Agricultural Organization of the United Nations and the International Atomic Energy Agency. Work conducted in collaboration with the University of Brussels and national laboratories in Africa and Asia has shown that the test to diagnose T. brucei can also be used to diagnose T. evansi infections in domestic livestock. Trypanosoma evansi is an economically important pathogen in Africa, Asia and Latin America. The efficacy of this assay in detecting T. evansi infections in buffalo, pigs and horses will be tested in Indonesia in a collaborative program run by ILRAD and research institutes in Indonesia and Australia.
Molecular probes to identify species
Our understanding of the epidemiology of both animal and human trypanosomiasis is improved by an ability to identify more precisely the trypanosome species carried by tsetse flies. To this end ILRAD has produced DNA probes to detect differences among parasite species and subspecies at the genomic level. These highly sensitive probes can be used in the laboratory to distinguish repetitive DNA sequences that recognize specific species and subspecies of pathogenic trypanosomes. The probes also distinguish differences among trypanosome populations of the same species collected from different areas. When the probes were tested on field materials collected in Kenya in collaboration with the International Centre of Insect Physiology and Ecology (Nairobi), it was discovered that although the probes are species-specific and detect low numbers of trypanosomes in infected tsetse flies, the probes do not detect all isolates of a given species. These results demonstrate that genetic differences occur within species; attempts will now be made to identify DNA sequences common to all populations of a given trypanosome species.
All the genetic probes in use today are radioactively labelled. Because most laboratories in Africa lack radioisotope facilities, these probes are not widely employed. Their use would be greatly promoted if the radioisotope were replaced with simple chemical groups that could be detected immunologically. ILRAD scientists have addressed this problem by producing derivatives of the probes with dinitrophenol so that positive hybridization using the repetitive sequence DNA probes can be revealed by an enzyme-labelled anti-dinitrophenol antibody. Although the reproducibility of the derivatization depends critically on the purity of dinitrobenzaldehyde, the initial labelling agent, dinitrophenol-labelled probes can detect as little as 1-5 pg of purified DNA, which demonstrates a sensitivity similar to that of the radiolabelled probes and probably sufficient for detecting trypanosomes in tsetse.
Future work at ILRAD in this area will attempt to validate the diagnostic assays and to increase their sensitivity where that is desirable. ILRAD staff will also work with scientific organizations to develop forms of the tests that can be widely and easily used in the areas of the world affected by trypanosomiasis.
Chemotherapy
One of the principal objectives of the work conducted at ILRAD on chemotherapy and chemoprophylaxis is to develop assays to quantify the levels of drugs in the blood and tissue fluids of treated livestock. Two kinds of such assays chemical and biological have been produced. The former, which requires sophisticated chemical techniques, will be used to quantify biological assays based on trypanosome culture systems. The simpler biological assays should prove useful to national laboratories.
In vitro assays of drug resistance
ILRAD scientists have shown that incorporating drugs in culture medium can inhibit the growth and development of trypanosomes in vitro, depending upon the sensitivity of the trypanosome population to the drugs used. Cultures of the bloodstream forms of the parasite were used in the first experiments in this area. Clear differences in Berenil-induced growth inhibition were observed (Figure 14). Bloodstream forms of fresh field isolates, however, are difficult to adapt to cultures. Cultures of procyclic forms of the trypanosome the noninfective, multiplying forms of the parasite equivalent to the forms found in the tsetse midget of T. brucei and T. congolense seem to be established more reliably, and tube assays have been developed for isolating and testing T. congolense and T. brucei for drug sensitivity.
Blood samples containing trypanosomes are mixed with a culture medium and incubated overnight at 27°C in a plastic centrifuge tube. The trypanosomes in the supernatant are removed and transferred to culture flasks to allow the procyclic forms to develop and grow further. All trypanosome stocks were successfully isolated by this procedure in a medium based on the composition of tsetse haemolymph to which supplements had been added.
The growth of procyclic forms of drug-sensitive trypanosome stocks was inhibited when these were propagated for ten days in the presence of 1 ng/ ml of isometamidium chloride. In cultures of sensitive trypanosomes, the drug induced an increase in cell size, degradation of the kinetoplast and multinucleate forms to develop. Differences in growth inhibition of procyclic forms between resistant and sensitive stocks also occurred after incubation for 48 hours with 1�100 ng/ ml isometamidium chloride. Only minor differences, however, were observed when procyclic forms of resistant and sensitive T. b. brucei were cultivated with diminazene aceturate. Thus, whereas inhibition of the growth of procyclic forms can be used to test trypanosome stocks for their levels of resistance to isometamidium chloride, the same assay appears to be inappropriate for diminazene aceturate.
An alternative method of testing trypanosome populations for resistance to both isometamidium chloride and diminazene aceturate is the drug incubation infectivity test. Cultures of trypanosomes are incubated at 37° C in 4% carbon dioxide in air for 24 hours in the presence of a drug or plasma from drug-treated animals. Control cultures incubated with 1 % distilled water instead of a drug are incubated under the same conditions. After incubation, an aliquot from each culture is inoculated into mice. The mice are then screened for 30 days for trypanosome infections.
None of the drug-sensitive trypanosome stocks were able to infect the mice after incubation with 1 ng/ ml isometamidium chloride, but all resistant stocks were able to do so. Two of the isometamidium-resistant trypanosome stocks retained their infectivity after incubation with 10 or 50 ng/ ml of the drug. But when used at higher concentrations on resistant trypanosome populations, the drug increased the period between infection and the appearance of parasites in the blood of the mice.
Higher concentrations of diminazene aceturate were necessary to inhibit the infectivity of T. b. brucei. Differences in infectivity occurred when trypanosomes were incubated in the presence of 0.05�1.00 µg/ ml of diminazene aceturate. The drug-resistant trypanosome stocks retained infectivity after incubation with 1 µg/ ml of the drug. Thus, by using the drug incubation infectivity test it is possible to distinguish isometamidium-and diminazene-resistant trypanosome populations from those that are sensitive to these drugs. The test has been validated for T. brucei, T. evansi and a rodent-infective stock of T. vivax.
The stability of the drug-resistant trait has been further examined in vitro: a T. brucei stock resistant to diminazene, isometamidium, quinapyramine and mel B was grown in vitro and its sensitivity to these drugs was compared to that of a drug-sensitive trypanosome stock. There was little change in sensitivity after propagation for up to 275 days of bloodstream forms in vitro or after transformation of bloodstream forms into procyclic, epimastigote and, finally, metacyclic forms. These results suggest that drug resistance is a relatively stable characteristic even when trypanosomes are maintained in vitro in the absence of drugs.
Significant progress was made in cultivating certain stocks of the Trypanozoon group without feeder-layer cells. This has enabled researchers to conduct several new studies, including an evaluation of trypanocides in the absence of feeder-layer cells.
Drug resistance
The development of resistance in trypanosomes to the trypanocidal drugs currently available threatens our ability to continue to control trypanosomiasis. In collaboration with the departments of Veterinary Parasitology and Physiology at the University of Glasgow, ILRAD is conducting studies to determine new ways of treating livestock that are infected with trypanosomes known to be resistant to recommended doses of trypanocidal drugs. Reports from the field have suggested that intravenous administration of isometamidium chloride has a curative effect on cattle infected with trypanosome populations that are resistant to the drug when it is administered intramuscularly. In experiments at ILRAD, however, the intravenous administration of high levels of isometamidium chloride failed to cure cattle infected with cloned populations of T. congolense that were resistant to this drug when it was administered intramuscularly.Using highly sensitive radiochemical and chemical techniques, a relay toxicity study, carried out in 1988 by the Department of Veterinary Pharmacology at the University of Glasgow in collaboration with ILRAD and the manufacturers of isometamidium chloride, Rhone-Poulenc (UK), showed that isometamidium chloride cannot be absorbed into blood and body fluids from the gastrointestinal tract of animals that were either dosed with the drug by mouth or fed with tissues of an animal given the drug intramuscularly one week previously. This makes it unlikely that human consumption of meat from animals treated with isometamidium chloride is a significant health hazard.
Tsetse biology
Male Glossina flies sexually sterilized by gamma-irradiation carry trypanosomiasis as efficiently as fertile males. However, when tsetse are given a blood meal containing 8�12�g/ml of isometamidium chloride before a blood meal containing trypanosomes, infection of the flies by mature T. vivax, T. congolense and T. b. brucei was completely suppressed. It is therefore recommended that the flies used in the sterile insect release method of tsetse control be fed on blood containing isometamidium chloride.Various other factors were investigated to determine their influence on the maturation of the three major species of trypanosomes and their transmission by tsetse. These parameters included the species of the wild mammalian hosts and tsetse vectors of the parasites and the occurrence of pre-existing trypanosome infections in the hosts and rickettsia-like organisms in the vectors (Figure 15). In ILRAD experiments, G. m. centralis appeared to be largely unaffected by these parameters and proved the most efficient vector of trypanosomiasis.
Figure 15. Electron micrograph of part of a midgut epithelial cell from a 30-day-old non-teneral Glossina morsitans centralis, showing the presence of large numbers of rickettsia-like organisms (R) throughout the cytoplasm. Magnification = ×5,300.
Further entomological research at ILRAD shows that the developmental cycle of T. vivax in tsetse, thought to be confined to the proboscis of the fly, may extend to the cibarial-oesophageal region, the most anterior part of the digestive tract of a tsetse. Tsetse proboscides were excised at intervals beginning one hour after an infected feed and transferred to culture dishes. Parasite multiplication and full cyclical development were observed only in proboscides excised four or more hours after the infected blood meal. It thus appears that in some tsetse, development of T. vivax through its vector stages is initiated in the cibarial/ oesophageal region, from where the parasites migrate to the food canal of the proboscis, where maturation to the metacyclic forms completes the life cycle of the parasite.
Early in 1988 a tsetse control campaign conducted by the African Trypanotolerant Livestock Network was started in Côte d'Ivoire using biconical traps impregnated with cypermethrin insecticide. Initial results indicate that this control method can reduce tsetse populations by more than 98%.
A comparison of tsetse blood meals at two sites in Zaire and one site in Gabon demonstrated that Glossina tabaniformis�a species of tsetse belonging to the fusca, or forest-dwelling group�takes a considerable proportion of feeds from cattle in these areas when this species comes into contact with the cattle. Because the fusca group inhabits forest zones of West and Central Africa, most of which are distant from cattle grazing areas, it had previously been thought that G. tabaniformis contributed little to trypanosome transmission.
Trypanotolerance
Trypanotolerant livestock, principally the N'Dama breed of cattle from West Africa, offer another means of improving agricultural productivity in tsetse-infected areas. ILRAD collaborates with the International Livestock Centre for Africa (ILCA [Addis Ababa]) and national livestock ministries and development programs in West and Central Africa in the African Trypanotolerant Livestock Network. With this network and the International Trypanotolerance Centre (the Gambia), ILRAD is studying the productivity of trypanotolerant livestock under different levels of trypanosomiasis risk and is seeking economically viable ways to increase the productivity of these livestock.ILRAD contributes to the network's activities by conducting collaborative programs on bovine genetics and trypanocide use and by helping national organizations in six countries apply standardized techniques in the collection of entomological and animal health data. A statistically significant relationship has been established at several sites between the monthly estimates of tsetse challenge and trypanosome prevalence in trypanotolerant cattle.
In a search for genetic markers of trypanotolerance, two polymorphic systems of bovine lymphocyte antigens were studied in 1988 in collaboration with ILCA. These systems are the major histocompatibility complex (MHC) and a more limited polymorphic system of common leucocyte antigens, which was detected in cattle only recently. The first objective of the study was to survey the MHC and common leucocyte antigen phenotypes of populations of N'Dama cattle in Zaire and the Gambia and to compare these phenotypes with corresponding profiles of trypanosensitive Boran cattle in Kenya. The second objective was to look for associations between these MHC and common leucocyte antigen phenotypes, trypanotolerance and the productivity of N'Dama cattle. Significant correlations have been found between the two classes of lymphocyte markers and the degree of resistance shown by trypanotolerant cattle exposed to trypanosomiasis by natural challenge. These provocative results, which suggest that there is a genetically selectable marker for the trypanotolerant trait, are being investigated further using larger numbers as well as family groups of cattle. The results also indicate a central role for immunity in the manifestation of the trypanotolerant trait.
ILRAD's embryo transfer experiments continued in 1988. N'Dama heifers produced at ILRAD from frozen N'Dama embryos brought in 1983 from the Gambia have since 1987 been regularly induced to superovulate using Folltropin or follicle-stimulating hormone derived from pigs. By implanting the best of the N'Dama embryos in Boran foster mothers, ILRAD has produced 24 N'Dama calves, which are used in studies of trypanotolerance and bovine genetics. ILRAD hopes to produce twin N'Dama calves by using these techniques so that research may be carried out on genetically matched animals.
The African Trypanotolerant Livestock Network will continue to evaluate the performance of trypanotolerant livestock in tsetse-infested areas of Africa, to seek ways to exploit the genetic resistance to trypanosomiasis in such livestock and to determine the most economic ways of improving the productivity of these livestock by nutritional and breeding strategies.
Long-term strategies for trypanosomiasis control
The biology of the trypanosome
The current methods used to control trypanosomiasis in cattle breeds other than N'Dama are highly susceptible to breakdown. The search for novel and more sustainable control measures for these breeds therefore forms the basis of ILRAD's long-term trypanosomiasis research program. Emphasis in this program is put on finding immunological ways to control the disease. Research is being conducted on the biology of the parasite in an attempt to discover processes unique to the parasite that could be the target of interventions. The aims of research on host-parasite interactions are to elucidate host mechanisms involved in controlling and destroying the parasite and then to enhance these mechanisms so as to reduce or alleviate the pathogenic manifestations of the disease. Research projects on basic parasite processes, such as the uptake of nutrients and the control of differentiation, were consolidated during the year and methods to cultivate trypanosomes were improved.Antigenic variation
The variable surface glycoproteins (VSGs), which make up the surface coat of the trypanosome, bear a complex lipid-containing structure at their carboxyl end called the glycosylphosphatidylinositol, or GPI moiety. Part of this structure is responsible for anchoring the VSG molecules in the plasma membrane of the trypanosome. Unlike the protein part of VSG molecules, which differs from one VSG to another, the hydrophobic GPI anchor is similar in many of the surface coat molecules examined from T. brucei and T. congolense. Antibodies can be raised in rabbits that will react with virtually all VSGs from these trypanosome species because the antibodies bind to a cross-reacting determinant (CRD) within the hydrophobic anchor and not to the variable, protein part of the VSG molecule.It has been suggested that the lipid portion of the anchor must be removed from membrane-form VSG (mfVSG) by an endogenous VSG-specific phospholipase C (GPI-PLC) before antibodies will bind to the CRD. Using T. congolense metacyclic forms from culture and substantiating their findings with bloodstream forms of T. congolense and T. vivax, workers at ILRAD have shown that the method of sample preparation is extremely important: the anti-CRD antibodies will bind to suitably prepared VSGs without prior removal of the lipid. However, the addition of dithiothreitol, which causes the protein part of the VSG molecule to partially unfold, was crucial in these experiments (Figure 16). It would seem, therefore, that a conformational change in the protein part of the VSG molecule is needed to expose the CRD to the antibodies before the antibodies can bind to the CRD.
Using density centrifugation of subcellular fractions of T. brucei, the enzyme responsible for removing the lipid from the hydrophobic anchor (the GPI-PLC) has been tentatively localized in a vesicle that contains both an internalized protein (bovine serum albumin) and an enzyme of the trypanosomal flagellar pocket (adenyl cyclase). The co-localization of these three reagents suggests that the GPI-PLC may reside in a vesicle near the flagellar pocket and that it may play an important role in recycling VSG.
At the molecular genetic level, metacyclic VSG genes of T. congolense IL 3000 have been cloned and expressed in the bacterium Escherichia coli. This has provided nucleotide sequence data for the VSGs of genuine metacyclic trypanosomes. The only material previously available for examination comprised bloodstream-form trypanosomes expressing cross-reactive VSGs. The genetic context in which these metacyclic VSG genes are expressed is now being examined. This is important because metacyclic trypanosomes express a constant but smaller set of VSGs than bloodstream forms belonging to the same repertoire.
Differences have already been observed between the two metacyclic VSG genes that have been cloned, mVSG1 and mVSG2. Only one copy of mVSG1 is conserved in all the developmental stages examined; at least two copies of mVSG2 exist in the metacyclic trypanosomes. Each of the metacyclic VSG genes is expressed at a different locus on chromosomes that are 2 megabases in size. One gene, mVSG1, is activated in situ; the other, mVSG2, is rearranged when it is expressed.
Work is in progress to determine the amino acid sequence and ancillary moieties of two VSGs from rodent-infective T. vivax clones. No peptide homology between the two VSGs has been demonstrated by using serological techniques and little information on the amino acid sequence has been obtained from analyses of purified VSGs. However, the importance of this work was manifested in 1988 with the discovery that, unlike the little antigenic cross-reactivity observed among geographically different populations of T. brucei or T. congolense, extensive antigenic cross-reactions occur among the variable antigen repertoires of cloned T. vivax populations derived from parasite populations isolated from widely different geographical locations across Africa. The cross-reactivity may be due to variable antigen genes shared among serodemes or to shared epitopes on the variable surface antigen molecules themselves. The assay used to analyse this cross-reactivity was immune lysis of bloodstream-form parasites. The assay demonstrates that the cross-reactivity occurs at the cell surface of living trypanosomes and not just between isolated molecules. Use of this assay has strengthened the view that the occurrence of cross-reactive antigens in T. vivax stocks contributes to the induction of immunity to this parasite species.
Differentiation
Although antigenic variation is the primary pathway used by the trypanosome to ensure its survival in the mammalian host, physiological mechanisms in the parasite that limit the numbers of parasites in the mammal also ensure trypanosomal survival by ensuring that some parasites are transmitted onward to the parasite's intermediate host, the tsetse fly. One of ILRAD's trypanosomiasis research areas focuses on genetic and biochemical work to identify the mechanisms in the parasite that control the parasite's proliferation and differentiation.The change in bloodstream T. brucei trypanosomes from actively dividing slender forms to non-dividing stumpy forms (Figure 17) is of particular interest to ILRAD since this switch may provide a clue to the regulatory genes and sequences responsible for the differentiation. The aim of research in this area is first to understand the mechanisms involved in the differentiation process and then to design artificial mechanisms that mimic the process.